Check Out Our Reference Guides on Radiation Safety
Types of Radiation
Ionizing Radiation
Non-Ionizing Radiation
Having the appropriate radiation shielding when working with radioactive material can greatly decrease the potential for exposures. It is also important to understand that certain shielding material should not be used for other radiations.
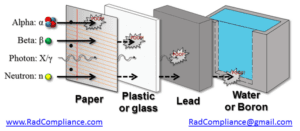
Examples of improper use include:
- Lead (high-Z material) should not be used to shield beta radiation because it can create bremsstrahlung radiation.
- Lead can be used for shielding alphas but so can paper (which is much cheaper)
Presented here is a simple list of popular isotopes (referred to as CHIPS) and their properties.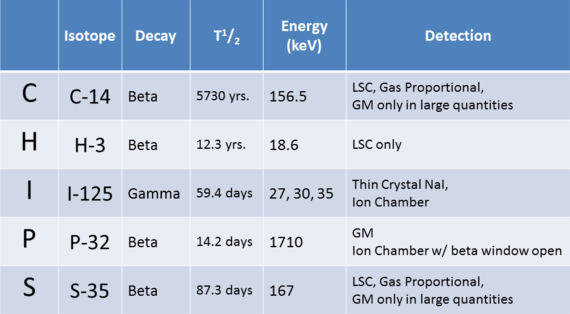
Not all radiation detectors can be treated as equal. The most important aspect of any detector is understanding its capabilities and limitations. Although we can help you choose the correct detector for your needs, following the information presented here can also be helpful.
- Determine whether you want to measure radiation exposure or radioactivity.
- Determine what configuration will meet your needs (portable, swipe counter, gamma spectroscopy, effluent, etc.)
- Compile a list of types of radiations (beta, gamma, etc.) and the isotope if possible, to determine the energy of the radiations that need to be measured.
- Research detector manufacturers and detectors capable of completing the task. Communicate with the manufacturer and even request a trial or demonstration of the device, if possible.
Radiation Detector Vendors (Some)
- Arrow-Tech, Inc.
- Berkeley Nucleonics
- Canberra Industries
- Direct Scientific
- F&J Specialty Products
- Femto-Tech, Inc.
- Gamma Products, Inc.
- Health Physics Instruments
- HI-Q Environmental Products
- Ludlum Measurements, Inc.
- Mirion Technologies
- ORTEC
- Quest Env. & Safety Products, Inc.
- SAPHYMO GmbH
- Spectrum Techniques, LLC
- Technical Associates
- Thermo Fisher Scientific
- Tracerco
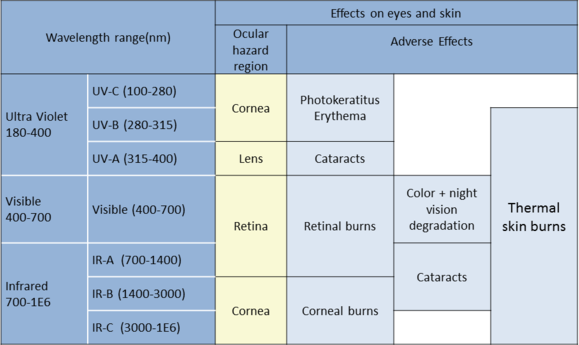
Laser is an acronym for Light Amplification by Stimulated Emission of Radiation. Lasers emit light on the electromagnetic spectrum that can be very useful but also hazardous if not controlled properly. Lasers are referenced as specific devices having the properties listed below from wavelengths of 180nm–1mm (ANSI Z136.1).
Lasers emit light that is:
- Directional: does not expand as easily as regular light (travels much further),
- Highly coherent: identical in wavelength and phase,
- Monochromatic: identical wavelength (very narrow band)